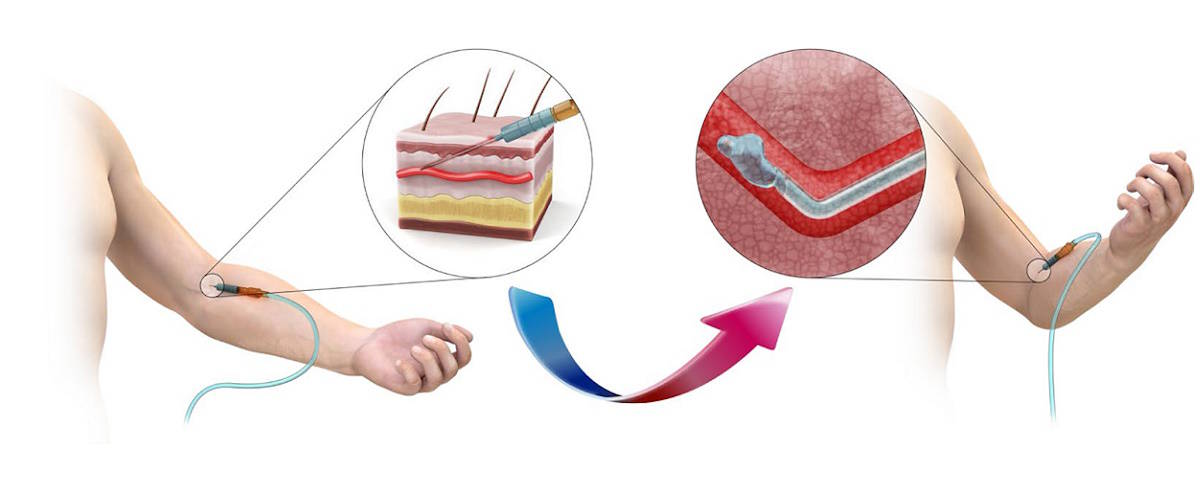
If a medical intravenous (IV) needle could soften upon insertion into the body, to match the softness of biological tissue, patients would experience less pain during drug injection, be more comfortable while receiving IV fluids, and have less risk of tissue damage to the blood vessel wall. A multi-disciplinary team of researchers in Korea has created just such a device.
The researchers, from the Korea Advanced Institute of Science and Technology (KAIST), are developing an IV needle with a rigidity and shape that depends upon body temperature. The phase-convertible, adapting and non-reusable, or “P-CARE”, needle is made of gallium (a soft metal with a melting point of 29.76 °C), which forms the hollow mechanical needle frame, encapsulated within a high-tear-strength soft silicone.
The needle is rigid at room temperature and can puncture soft biological tissue. Once inserted into the body, however, it becomes soft and flexible due to the higher local tissue temperature and can dynamically adapt to tissue deformation while reliably delivering fluid. Importantly, the needle maintains its soft state upon removal from the body, preventing inappropriate reuse or accidental needlestick injuries.
In rigid mode, the P-CARE needle has high bending stiffness and an insertion force similar to that of a standard 18 gauge IV catheter. It has a maximum achievable flow rate comparable to that of a standard 22 gauge IV catheter and can reliably deliver fluid up to a bending radius of 5 mm. Once in softened mode in the vein, the P-CARE needle’s curvature is unaffected by the flow rate of the fluid being delivered through the inner channel (when using typical flow rates of standard IV catheters).
Writing in Nature Biomedical Engineering, the researchers explain that stiffness-tuning with superior tissue adaptability is a unique feature of the P-CARE needle. “[This] overcomes the fundamental limitation of the conventional IV access devices, which have intrinsically high and fixed stiffness and can lead to tissue trauma at the injection site, particularly when the patient moves, because of the large mechanical mismatch between the rigid needle and soft tissue,” they write.

Co-principal investigators Jae-Woong Jeong and Won-Il Jeong and colleagues also integrated a thin-film temperature sensor into the P-CARE needle for in-body temperature sensing. During an IV medication, hospitalized patients can experience changes in their core body temperature or unintended leakage of infused fluid in the subcutaneous layers. Such conditions must be properly monitored as they may lead to complications that require additional medical procedures. Through in vivo mice studies and ex vivo experiments in porcine muscle tissue, the researchers demonstrated the reliable on-site temperature-sensing ability of the P-CARE needle.
Mechanically guided needle eases difficult injections
The team also performed 14-day in vivo biocompatibility studies in mice. Results showed that P-CARE needles caused significantly less inflammation than similar-sized standard IV access devices made of metal needles or plastic catheters when inserted into muscle tissue. The P-CARE needle also was able to deliver medications as reliably as the commercial IV access devices.
The researchers hope that the softening IV needle will be translated for clinical application. This will require several systematic studies, the first to establish the level of safety of the device, benchmarked to US Food and Drug Administration (FDA) requirements. The team is also continuously enhancing the design, structure and packaging of the needle, to further evaluate its long-term reliability and safety of use in the blood vessel.