A new type of computer model that can reveal radiation damage at the cellular level could improve radiotherapy outcomes for lung cancer patients.
Roman Bauer, a computational neuroscientist at the University of Surrey in the UK, in collaboration with Marco Durante and Nicolò Cogno from GSI Helmholtzzentrum für Schwerionenforschung in Germany, created the model, which simulates how radiation interacts with the lungs on a cell-by-cell basis.
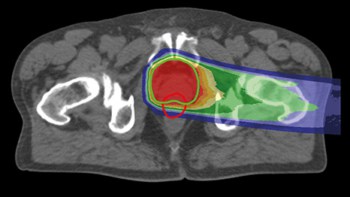
Over half of all patients with lung cancer are treated using radiotherapy. Although this approach is effective, it leaves up to 30% of recipients with radiation-induced injuries. These can trigger serious conditions that affect breathing, such as fibrosis – in which the lining of the alveoli (air sacs) in the lungs is thickened and stiffened – and pneumonitis – when the walls of the alveoli become inflamed.
In order to limit radiation damage to healthy tissue while still killing cancer cells, radiotherapy is delivered in several separate “fractions”. This allows a higher – and therefore more effective – dose to be administered overall because some of the damaged healthy cells can repair themselves in between each fraction.
Currently, radiotherapy fractionation schemes are chosen based on past experience and generalized statistical models, so are not optimized for individual patients. In contrast, personalized medicine could be achieved thanks to this new model which, as Durante, director of the Biophysics Department at GSI explains, looks at “toxicity in tissues starting from the basic cellular reactions and [is] therefore able to predict what happens to any patient” when different fractionation schemes are chosen.
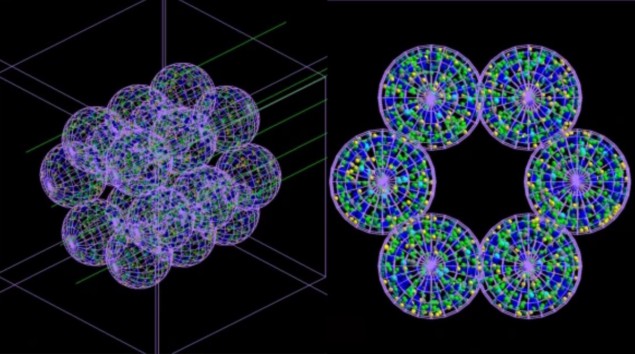
The team developed an “agent-based” model (ABM) consisting of separate interacting units or agents – which in this case mimic lung cells – coupled with a Monte Carlo simulator. The ABM, described in Communications Medicine, builds a representation of an alveolar segment consisting of 18 alveoli each 260 µm in diameter. Next, Monte Carlo simulations of irradiation of these alveoli are carried out at the microscopic and nanoscopic scale, and information about the radiation dose delivered to each cell and its distribution is fed back into the ABM.
The ABM uses this information to work out whether each cell would live or die, and outputs the final results in the form of a 3D picture. Crucially, the coupled model can simulate the passage of time and thus show the severity of radiation damage – and the progression of the medical conditions it may cause – hours, days, months or even years after treatment.
“What I found very exciting is that these computational simulations actually delivered results that matched with various experimental observations from different groups, labs and hospitals. So our computational approach could in principle be used within a clinical setting,” says Bauer, the spokesperson for the international BioDynaMo collaboration, which aims to bring new computational methods into healthcare via the software suite used to build this model.
Bauer began working on computational cancer models after a close friend died from the disease aged just 34. “Every cancer is different and every person is different, with different shaped organs, genetic predispositions and lifestyles,” he explains. His hope is that information from scans, biopsies and other tests could be fed into the new model to provide a picture of each individual. An AI-assisted therapy protocol could then be created that would output a closely tailored treatment plan that improves the patient’s chances of survival.
Patient-specific planning could improve radiotherapy outcomes
Bauer is currently seeking collaborators from other disciplines, including physics, to help move towards a clinical trial following lung cancer patients over several years. Meanwhile, the team intends to expand the model’s use into other areas of medicine.
Durante, for instance, is hoping to study viral infection with this lung model as it “may predict the pneumonitis induced by the COVID-19 infection”. Meanwhile, Bauer has begun simulating the development of circuits in the brains of premature babies, with the goal of better understanding “at what time point to intervene and how”.