
The goal of radiotherapy is to deliver a prescribed radiation dose to the tumour target while limiting damage to surrounding normal tissues. This is currently achieved using population-based treatment plan optimization, based on predefined dose-based objectives and organ-at-risk (OAR) constraints developed from the aggregated response to radiation of a broad patient population. Unfortunately, the effectiveness and toxicity of such standardized treatment plans vary, because patients and their tumours have individual biological characteristics.
Aiming to provide a more personalized approach to radiotherapy planning, researchers at the University of Michigan have developed a novel intensity-modulated radiotherapy (IMRT) optimization strategy that directly incorporates patient-specific dose-response models into the planning process. Their technique, described in Medical Physics, is based on maximizing the predicted value of overall treatment utility – defined as the probability of local control minus the weighted sum of toxicity probabilities.
The new planning method, called prioritized utility optimization (PUO), augments standard approaches by incorporating personalized factors related to the radiosensitivity of tumours and OARs. OAR radiotoxicity, for instance, can be influenced by age, smoking status, gene expression, molecular markers and pre-existing conditions such as cardiac disease. Other concurrent treatments may also impact the efficacy of radiation therapy.

To validate their strategy, principal investigator Martha Matuszak and colleagues used the PUO method to create IMRT plans for five patients with non-small cell lung cancer (NSCLC). They report that PUO planning improved local control for all patients compared with the conventional plans that had been used for their treatments.
“NSCLC patients represent a highly heterogeneous group with variability in extent and localization of disease,” explains lead author Daniel Polan. “In combination with other anatomic variability, these factors can drastically impact treatment planning, including any anticipated gains from differing optimization methods. Therefore, for initial feasibility testing of our method, we selected five cases to represent diversity in patient size, tumour size, location and laterality, in addition to diversity in dose covariates influencing predicted outcomes.”
To create patient-specific IMRT plans, the researchers first used a commercial treatment planning system to calculate dose based on an influence matrix of beamlet-dose contributions to regions-of-interest. They then solve two optimization problems to generate optimal beamlet weights that can be imported back into the TPS.
The first optimization problem maximizes the overall plan utility subject to typical clinical dose constraints, by optimizing the trade-off between efficacy and toxicity based on individualized dose-response models. The second minimizes conventional dose-based objectives, subject to the same dose constraints as the first, while maintaining the optimal utility determined from the first optimization.
For all five patients, the PUO approach successfully generated optimal beamlet weights that maximized utility while remaining within dose-based constraints. For the study, the researchers compared these PUO IMRT plans with the clinically delivered 3D conformal radiotherapy (CRT) plans, and with retrospectively generated dose-only optimization (DOO) IMRT and volumetric-modulated arc therapy (VMAT) plans.
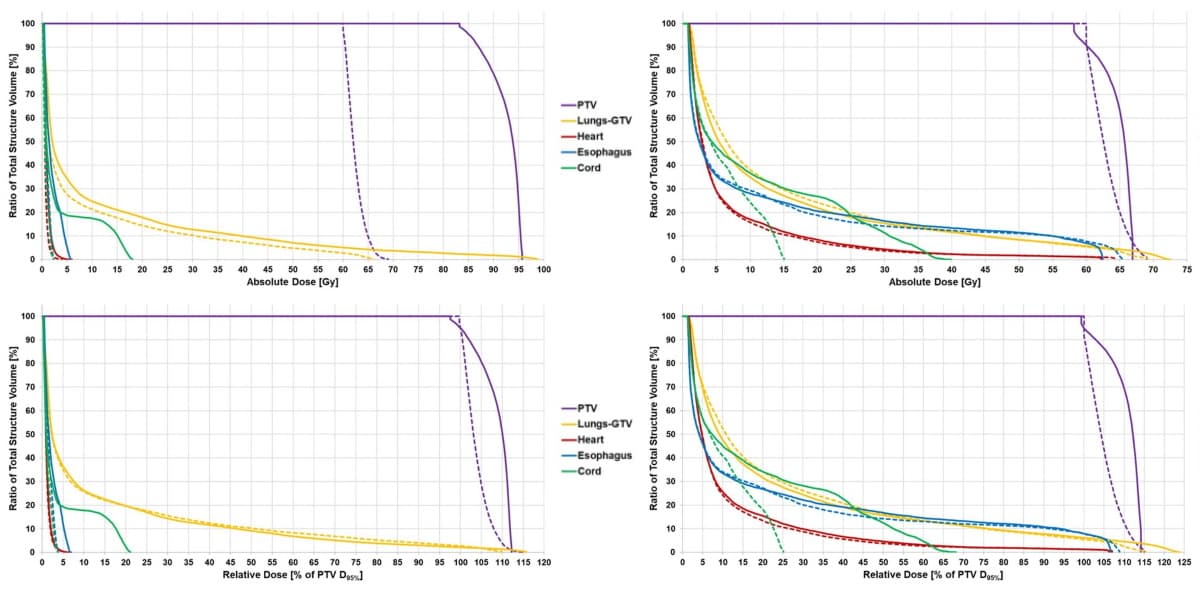
When compared with the 3DCRT, VMAT and DOO IMRT plans, the PUO method improved plan utility by an average of 40%, 32% and 31%, respectively. The PUO plans demonstrated an average 17% improvement in local control with similar toxicity to conventional planning.
As anticipated, the extent of benefits from the PUO IMRT plans differed among patients. Polan reports that for one patient, PUO resulted in a utility improvement of 70% over conventional DOO. “This corresponds to a 32% absolute improvement in the predicted probability of progression-free survival, while only increasing the predicted probability of radiation-induced lung toxicity by 2%,” he says. “This substantial trade-off has the potential to greatly improve disease survivability while minimizing the impact to a patient’s post-treatment quality-of-life.”
For another patient who had a large tumour, however, improvements were minimal. Polan explains that for larger tumours, treatment planning typically becomes more constrained due to increased integral dose requirements and a decreased ability to avoid bordering normal tissues.

AI framework uses medical images to individualize radiotherapy dose
The team emphasizes that the PUO method provides a quantitative way to determine which patients may benefit from dose escalation or redistribution, based on patient-specific clinical factors and biomarkers, while also accounting for patient geometry and OAR dose limits.
The researchers are currently conducting large-scale retrospective studies with the goal of developing a prospective clinical trial employing the PUO treatment planning strategy. Their research centres around integrating patient data and personalized outcome predictions directly into radiotherapy planning, with a current focus on liver, lung and head-and-neck cancers, where balancing the positive and negative effects of radiotherapy could significantly impact a patient’s overall quality-of-life.



