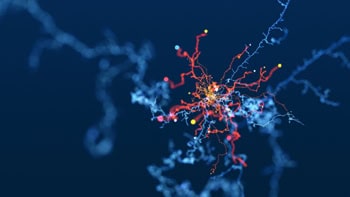
Scientists at the Universities of Vienna, Austria and Helsinki, Finland have captured the first direct images of clusters of room-temperature noble gas atoms by confining them in a “sandwich” made from two layers of graphene. Taken using a transmission electron microscope, the images could aid fundamental condensed-matter physics research and might have applications in quantum technology.
Led by physicist Jani Kotakoski, the team obtained the images while studying how radiation modifies the properties of graphene (a sheet of carbon just one atom thick) and other two-dimensional materials held together by weak van der Waals interactions. The scientists noticed that when they used noble gas ions to irradiate a sample of multilayer graphene, the ions could become trapped between two sheets of the material. For this to happen, the energy of the irradiating ions had to be just right: fast enough to pass through the first sheet, but not the second.
“We succeeded in doing this by implanting the noble gas ions into the multi-layered structures,” explains team member Manuel Längle, who began working on this project during his master’s thesis in late 2017. “If we find the implanted ions in a five-layer but not a two-layer sample, we know that the energy is too high.”
In their work, which is published in Nature Materials, the researchers studied krypton and xenon ion clusters using scanning transmission electron microscopy (STEM). They found that for krypton-irradiated samples, successful implantation between two graphene layers occurred at 60 eV. For xenon-irradiated samples, the “sweet spot” was between 55 eV and 65 eV.
Densely-packed two-dimensional nanoclusters
Because noble gases are mostly inert and seldom form chemical bonds, the atoms can move about freely within their graphene sandwich. In certain regions, however, two or more atoms can come together and form regular, densely-packed two-dimensional nanoclusters. These nanoclusters make an excellent testbed for studies of very weakly-interacting systems.
The researchers found that clusters of xenon made up of up to 100 atoms behave like solid systems but that krypton clusters containing as few as 16 atoms sometimes show fluid-like behaviour. Though they do not yet understand why, they say the finding could open a new field of study focused on encapsulated van der Waal materials.

Single atoms swim inside a graphene sandwich
According to Längle and Kotakoski, applications for these structures are difficult to predict at present. However, since noble gases are routinely employed in light sources and lasers, they might have some future use in quantum information technology.
Looking forward, the Vienna-Helsinki team now plans to repeat the experiments at different temperatures and pressures. “We also plan to study mixtures of gases and look into different two-dimensional materials like hexagonal boron nitride (sometimes called ‘graphene’s cousin’) or multi-layered structures,” Längle tells Physics World.