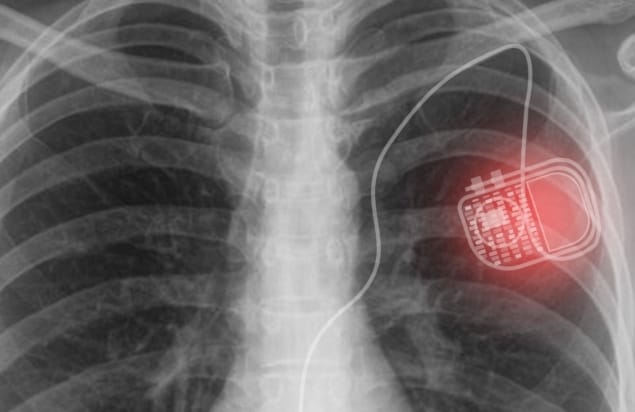
Biomedical electronic implants, such as cardiac pacemakers, deep brain stimulators or spinal cord stimulators, enhance quality-of-life by providing diagnostics and treatments within the human body. Most of these devices, however, are powered by batteries. And once these batteries run down, patients must undergo invasive surgery to replace them.
To address this obstacle, researchers at Gwangju Institute of Science and Technology (GIST) propose a new way to provide sustainable electrical power within the body without the risks of surgical complications – via a concept called active photonic power transfer. They have developed a power transfer system comprising a skin-attachable light-source patch and a photovoltaic array integrated into a flexible medical implant.
The thin, flexible micro-LED patch emits photons that penetrate through tissue and are captured by the photovoltaic array, which then generates electrical energy to power the implanted device. The authors note that unlike other implanted photovoltaics that rely on ambient light, this system can generate power indoors or outdoors, day or night and regardless of covering by clothes.
“One of the greatest demands in biomedical electronic implants is to provide sustainable electrical power for extended healthy life without battery replacement surgeries,” explains lead author Jongho Lee. “Currently, a lack of a reliable source of power limits the functionality and performance of implant devices. If we can secure enough electrical power in our body, new types of medical implants with diverse functions and high performance can be developed.”
Power patch
Lee and colleagues created the light-source patch from an array of AlGaInP micro-LEDs powered by a conventional 3 V battery. The micro-LEDs emit light at 670 nm, which penetrates roughly 2.5 mm through tissue and can be converted into electricity by photovoltaic materials such as GaAs (which has a bandgap lower than the photon energy).
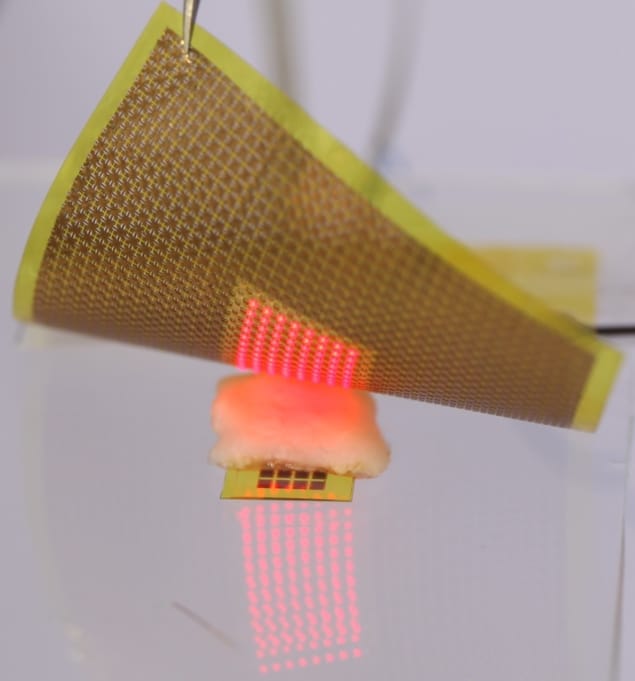
The researchers first demonstrated the patch’s thermal and mechanical compatibility with skin samples. Then to investigate its potential for power transfer, they tested the system in live mice. They fabricated a tiny (1 × 0.65 cm) flexible stimulator comprising a 4 × 8 array of GaAs photovoltaic devices, with a total active area of 11.1 mm2, and a small rechargeable microbattery. They implanted the stimulator through an incision in the animal’s skin and placed the light-source patch on the skin surface.
When the researchers switched on the light-source patch, the emitted photons successfully penetrated the animal’s tissues and wirelessly transferred power to the implanted stimulator. The power transmitted through the skin was 8.2 μW – greater than the required power consumption of the custom-built stimulator (roughly 2.3 μW). This power was enough to generate periodic stimulating pulses, as well as to charge the built-in microbattery. They note that even after turning off the light-source patch (at 106 min), the stimulator operated for an extra 90 min, powered by the charged battery.
The team also demonstrated that the implanted stimulator could regulate the heartrate of a mouse in bradycardia (a slower than normal heart rate) whilst wirelessly powered by the skin-attached patch. The stimulator generates pulses with a frequency of 3.3 Hz. As soon as the stimulator’s output lead wires were brought into contact with the right atrium and left ventricle, the animal’s heart started to beat regularly at around 3.3 Hz.
Commercial cardiac pacemakers require a power of between 1 and 10 μW, depending on operating mode. The researchers point out that that the power transferred by their custom-fabricated optoelectronic device (with an active photovoltaic area of 11.1 mm2) is already within this range, and that a pacemaker requiring 10 μW would need a minimum active area of 13.5 mm2.
“Currently, we are planning to develop the technology further to apply it for use in humans,” Lee tells Physics World. “We have to check or improve the mechanical reliability, long term biocompatibility and power density.”
Lee and colleagues present their findings in PNAS.