
Radiation is known to be damaging to the immune system, with recent studies linking radiation-induced lymphopenia (loss of lymphocytes, the white blood cells associated with immune response) with poor survival after radiotherapy. To better understand how radiation treatments affect circulating lymphocytes, researchers at MGH/Harvard Medical School developed a 4D computational blood flow model to calculate the blood dose during radiotherapy (Phys. Med. Biol. 10.1088/1361-6560/ab6c41).
“The blood flow model aims to estimate the ionizing radiation dose to the circulating blood during a course of fractionated radiotherapy,” says first author Abdelkhalek Hammi. “This will improve our understanding of the suppressive effects of radiation on the patient’s immune system and the emergence of radiation-induced lymphopenia,”
Hammi and colleagues developed an intracranial blood flow model based on major cerebral vasculature extracted from patient MRI data, and extended with a network of generic brain vessels. For blood distribution outside the brain, they developed the model according to the reference human body model. The cerebral model includes 1050 vascular path lines and simulates more than 266,000 blood particles; the model for the entire body contains 22,178,000 particles.
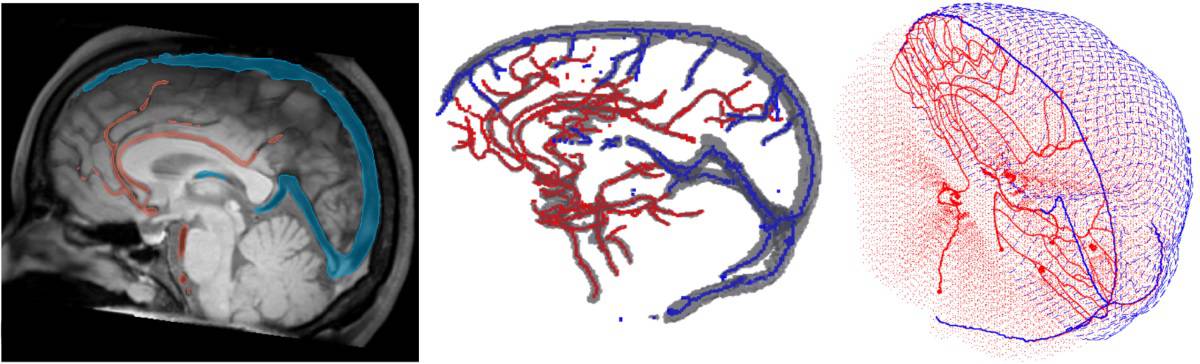
To determine the dose to the circulating blood, the team use Monte Carlo simulations to track the propagation of each individual blood particle through the brain and the time-dependent radiation fields. In a single treatment fraction, the blood dose approximates the dose to the circulating lymphocytes. For treatments spanning several weeks, however, Hammi notes that the effects of lymphocyte repopulation and exchange cannot be ignored, and will be investigated in future studies.
For clinical use, the model would use patient-specific input data. “The model itself will not change, but parameters such as treatment fields and the exact time structure of delivery will change,” Hammi explains. “In addition, the model parameters can be adjusted to account for the patient’s age, gender, blood pressure and other haemodynamic parameters that affect how the blood particles move between compartments.”
Comparing modalities
The researchers used their model to generate blood dose–volume histograms (DVHs) after intracranial intensity-modulated radiotherapy (IMRT) and passive scattering proton therapy. They created IMRT and proton plans for the same patient and target volume, simulated 30 treatment fractions (2 Gy per fraction, at 2 Gy/min) and calculated blood DVHs for each modality.
After the first fraction, the calculated mean dose to the blood pool was 0.002 Gy for proton therapy and 0.004 Gy for IMRT. After 30 fractions, the mean doses were 0.061 Gy for protons and 0.133 Gy for IMRT – an integral dose difference of more than 118%. The highest doses to 1% of blood after 30 fractions were 0.196 and 0.343 Gy, for protons and IMRT, respectively.
The volumes of blood receiving any dose after one fraction were 10.1% and 18.4%, for proton and photon treatments, respectively. Approximately 90% of the blood pool will have been irradiated after the 11th fraction of IMRT, but only after the 21st fraction of proton therapy.
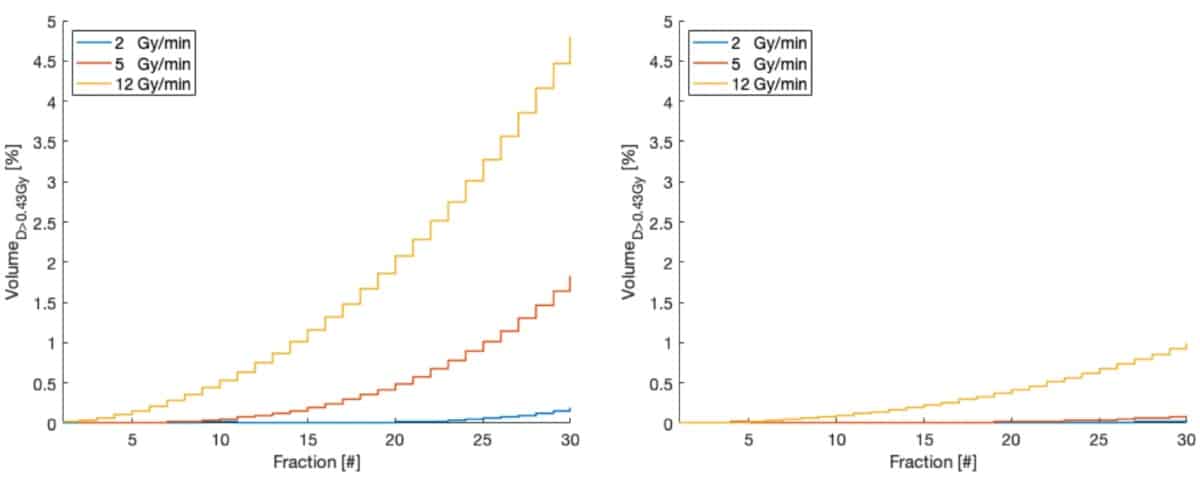
With a view to optimizing treatment parameters, the team investigated impact of dose rate on the dose to circulating blood. While dose rate did not affect the mean dose to the blood pool, an increased dose rate (and thus shorter beam-on time) reduced the fraction of blood receiving a low dose, but increased the volume receiving high doses.
For IMRT, increased dose rates did not significantly decrease the fraction of irradiated blood after 30 fractions: 97.7% and 94.2% at 5 and 12 Gy/min, respectively. For proton therapy, on the other hand, higher dose rates reduced the irradiated blood at the end of treatment: increasing the dose rate (from 2 Gy/min) to 5 and 12 Gy/min reduced the fraction of blood receiving any dose from above 95% to 78.8% and 60%, respectively.
The team also quantified the fraction of blood receiving more than a threshold of 0.43 Gy (the dose thought to lower CD8 lymphocyte count by 10%), and saw that higher dose rates increased the volume of blood receiving this dose. Proton therapy, however, irradiated five times less volume to this threshold dose than IMRT.
Finally, the researchers examined whether patient-specific variables such as cardiac output, gender and age affected the blood DVH. Higher cardiac output increased the blood volume receiving low doses and decreased the volume receiving high doses, mainly due to higher flow rate. They noted small differences between male and female patients (up to 4.3%), mainly due to differences in total blood volume and cardiac output. In younger patients, up to 10% more of the circulating blood received a low dose, independent of gender and treatment modality.
Young investigators focus on brachytherapy, dose modelling and deep learning
The researchers conclude that their blood flow model effectively estimates the dose to circulating blood during cranial radiation therapy. They showed that using proton therapy and increasing the dose rate can reduce the volume of irradiated blood.
“We are now working on end-to-end validation of the model based on measured lymphocyte depletion in patients treated with radiotherapy,” says Hammi. “We also want to extend the model to other organs and treatment sites.”



