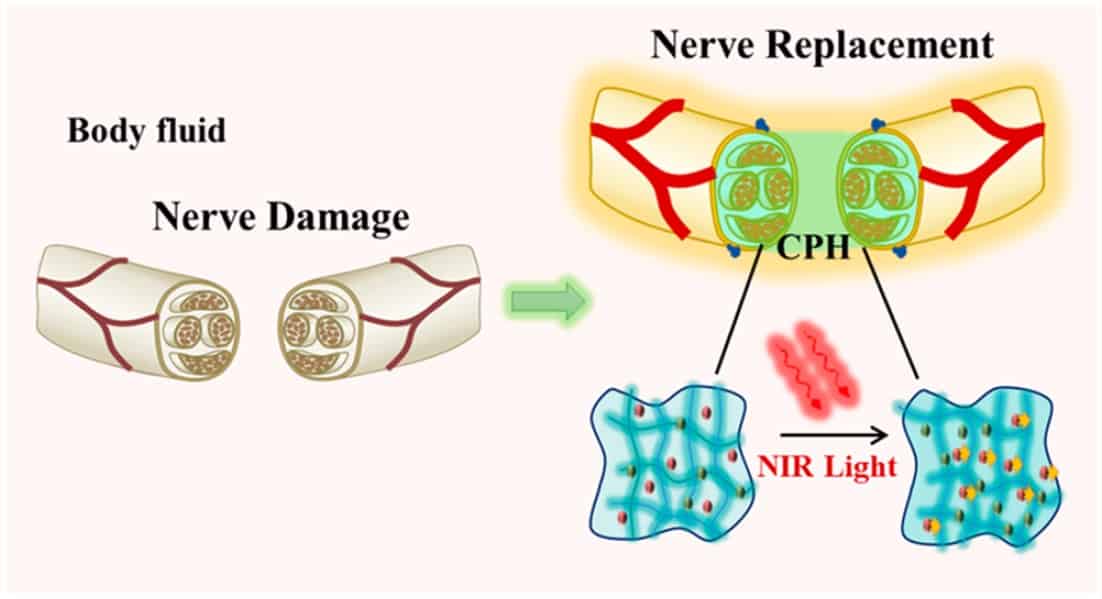
Researchers in China have developed a stretchable and conductive hydrogel that they claim could one day be used to repair peripheral nerves – delicate tissues that transmit bioelectrical signals from the brain to the rest of the body in real time. The hydrogel, which has been tested in rats with sciatic nerve injuries, remains electrically conducting when elongated and its conductivity improves when it is illuminated with near-infrared light. These two properties mean that it could be used to treat serious peripheral nerve injury, especially when the missing nerve length exceeds 10 mm.
Flexible electronics has come along in leaps and bounds in the last few decades, allowing bioelectronic materials to be used as artificial tissue in vivo. Hydrogels – 3D polymer networks that can hold a large amount of water – are similar in structure to nerve tissue, and interfacing these materials to living tissue is one of the most important topics in bioelectronics today.
Repairing injured peripheral nerves
Peripheral nerve injury – for example, when a peripheral nerve has been completely severed in an accident – can result in chronic pain, neurological disorders, paralysis and even disability. Such injuries, however, are difficult to treat.
One of the main techniques used to repair injured peripheral nerves is autologous nerve transplantation. This involves removing a section of peripheral nerve from elsewhere in the body and “sewing” it onto the ends of the severed nerve. There are nevertheless some shortcomings associated with this approach, including the fact that the surgery doesn’t always restore nerve function and that multiple follow-up procedures are sometimes required. There is also the risk of painful neuroma (benign growth of nerve tissue) after the operation.
Another technique relies on tissue engineering to restore and repair motor and sensory function of neuronal cells. This method makes use of natural or synthetic materials that can be grafted onto nerve cells together with “supporting” cells, such as mesenchymal stem cells. The problem with this approach is that the grafted nerves recover slowly.
Hydrogel conducts bioelectric signals
The research team – led by Qun-Dong Shen of Nanjing University, Chang-Chun Wang of the Nanjing Institute of Technology and Ze-Zhang Zhu of the Affiliated Drum Tower Hospital of Nanjing University – has now developed an alternative technique. In their work, the researchers made use of a mechanically tough but stretchable conductive hydrogel containing biocompatible polymers: polyacrylamide and conductive polyaniline. These crosslinked polymers boast a 3D microporous network that, once implanted, allows nerve cells to enter and adhere, helping to restore lost tissue.

The hydrogel conducts bioelectric signals – something that the team proved by replacing a damaged sciatic nerve from a toad with the material and measuring the signals through it. They also implanted the hydrogel into rats with sciatic nerve injuries. In these experiments, they observed that the rats’ nerves recovered their bioelectrical properties – as measured by electromyography one to eight weeks following the operation – and that their walking improved compared with rats that hadn’t been treated with the hydrogel.
Another advantage of the hydrogel is that the current flowing through it increases from 1.95 nA to 2.3 nA when it is irradiated with near-infrared light, which can penetrate deep into biological tissue. This proves that the hydrogel is relatively sensitive to light of this wavelength, say the researchers, and that the bioelectrical signal through the material can be increased in this way, so allowing for improved nerve conduction and recovery.
And that is not all: when elongated mechanically, the material remains conducting – just like biological nerve tissue. This means that it can accommodate the large strains produced by sutured nerve tissue in motion.
Full details of the research are published in ACS Nano.



