The primary goal of radiotherapy is to deliver a large radiation dose to cancer cells whilst sparing surrounding healthy tissue. Recent developments have shown that through delivery of ultrahigh dose rates to cancerous tissue, a technique known as FLASH radiotherapy, healthy tissue toxicity can be reduced, thereby improving the therapeutic ratio.
One approach for delivering the ultrahigh dose rates required for FLASH is to use proton therapy. As protons traverse through tissue, they deposit the majority of their energy at the end of their range. By ensuring maximum dose delivery within a confined volume, the combination of proton therapy and FLASH could improve the therapeutic ratio further.
Prior to treatment delivery, rigorous computational processes within the treatment planning system (TPS) help to determine the optimal plan. This optimization process also considers how to divide the total dose into multiple treatment fractions – known as hyperfractionation.
Currently TPS optimization algorithms only optimize the dose without consideration of the dose rate. However, the delivered dose rate has a significant impact on the efficacy of FLASH radiotherapy. To address this, Hao Gao and colleagues at Winship Cancer Institute of Emory University and Shandong University have developed a method for simultaneous dose and dose-rate optimization (SDDRO).
Dose-rate optimization
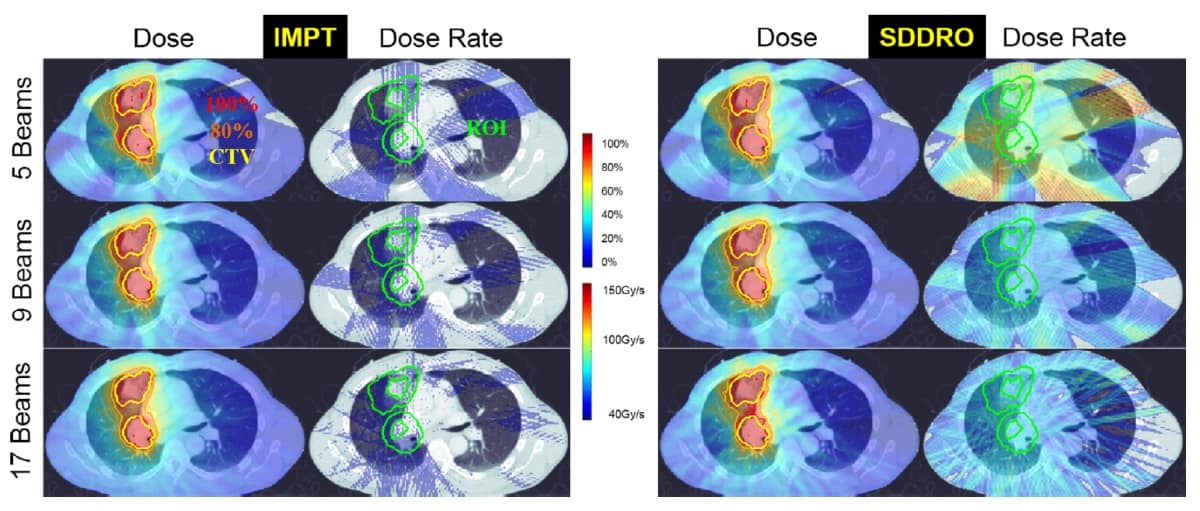
Gao and his team investigated the impact on plan quality when considering only dose optimization and when using SDDRO. They compared the dose and dose-rate distributions produced by SDDRO with traditional intensity-modulated proton therapy (IMPT) plans (with dose optimization only) for three lung cancer patients. They considered treatments delivered using one, three, five, nine and 17 beams, with fraction prescription doses of 2, 6 and 10 Gy.
The SDDRO method incorporates the usual dose constraints to the target volume and organs-at-risk (OAR). Additional dose-rate constraints are enforced, similarly to the dose constraints, in the region-of-interest (ROI). The ROI is selected as a ring-like expansion around the clinical target volume (CTV). The dose-rate constraints ensure that a large percentage of the ROI receives the desired FLASH dose rate (40 Gy/s or more).
Improved dose-rate coverage
In comparison to dose and dose-rate distributions produced by IMPT planning, SDDRO displayed significant improvements in the FLASH dose-rate coverage. The dose-rate constraint, where 98% of the ROI should receive the desired dose rate, was satisfied for all SDDRO plans in all cases. When multiple treatment beams were considered during optimization, the overall plan quality was improved, in terms of both dose and dose-rate distributions.
The best FLASH dose-rate coverage was obtained when planning treatments with nine beams and 10 Gy fractions. This suggests that the plan quality of SSDRO could be improved further by increasing the dose delivered per fraction – an approach known as hypofractionation.
The researchers also showed that the dose distributions produced with and without dose-rate optimization had comparable CTV coverage. Gao believes that “SDDRO can substantially improve the FLASH dose-rate coverage compared to IMPT for the purpose of normal tissue sparing while preserving the dose distribution”.
Future implementation of SDDRO
The ability of the proposed SDDRO method to handle dose-rate constraints is clear. For future implementation of this method, Gao suggests that “the dose rate–volume constraints should be prescribed in the similar fashion as the dose–volume constraints”.
While the authors acknowledge that implementation of the SDDRO method requires further development, they believe that this method may in the future become routine for FLASH treatment planning. “Unlike dose–volume constraints, for which various quantitative metrics have been established corresponding to clinical endpoints, the dose rate–volume constraints are new, for which the quantitative metrics are to be established,” says Gao.
The researchers report their findings in Medical Physics.