The ongoing COVID-19 pandemic has highlighted just how important it is to be able to accurately detect the tiny virus particles that cause diseases. A new method, recently described in Nature, can rapidly indicate the presence of proteins from a coronavirus and can be easily adapted to detect a wide variety of other important biological molecules.
The gold-standard of coronavirus testing is currently RT-PCR (real-time polymerase chain reaction) – a lab technique that boosts the presence of the genetic information of a virus to a level at which it can be detected. The pandemic has shown the weaknesses of this method: it requires specialized lab equipment and staff, while also using specific supplies that have been in short supply. Researchers led by David Baker from the University of Washington have produced an innovative biosensor that offers a completely new approach for COVID-19 diagnosis.
Opening the LOCKR
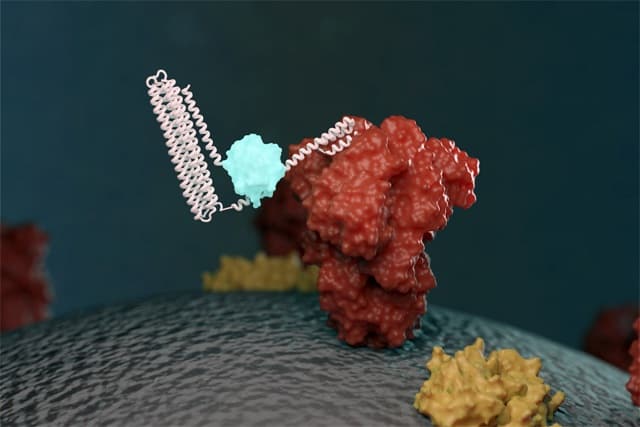
A biosensor takes an element from a living cell – often a protein – and uses it to enable the detection of a molecule of interest. These can be very useful and sensitive, but designing specific sensors for the task at hand can take considerable time and effort. To speed up the process, Baker and his colleagues have developed the LOCKR system – a template for producing a biosensor where the targeted molecule can be changed easily without having to redesign the entire system from scratch.
The biosensor utilizes light to indicate the presence of the target molecule. The LOCKR proteins initially exist in a “closed” state where they do not emit light. If the target is present, it will attach to a specific binding region of the sensor, causing LOCKR to switch to its “open” state and emit light. This allows the observer to easily see that the target has been found.
The key feature of the LOCKR system is that it can easily be adapted to sense a range of targets, because the target binding region can be swapped without affecting the rest of the system. This saves a lot of effort compared with building a brand-new biosensor. The other components can also be subtly tuned to find the appropriate sensitivity for the user’s intended application.
A world of possibilities
The research team showcased this adaptability by producing sensors for six different biologically interesting targets with the correct sensitivity for each target. They then used the sensors to detect both part of the SARS-CoV-2 virus and the antibodies produced to fight against COVID-19 infection – two testing applications that have been in large demand during the ongoing pandemic.
The biosensor was so effective it could detect concentrations of the SARS-CoV-2 virus as low as 15 picomolar – equivalent to being able to detect a single grain of salt dissolved in over 300,000 litres of water. The biosensors designed to detect specific antibodies against the SARS-CoV-2 virus were able to signal the presence of low concentrations of these antibodies after just a couple of minutes.
“We have shown in the lab that these new sensors can readily detect virus proteins or antibodies in simulated nasal fluid or donated serum,” says Baker. “Our next goal is to ensure they can be used reliably in a diagnostic setting. This work illustrates the power of de novo [from the beginning] protein design to create molecular devices from scratch with new and useful functions.”