Mosquitoes are often considered the world’s most dangerous animal. While feeding on blood through the skin they transmit pathogens that cause deadly diseases such as malaria, dengue, Zika and yellow fever. According to the World Health Organization, mosquito-borne diseases are responsible for more than 700,000 deaths each year.
Studying mosquito feeding behaviour could help develop countermeasures to decrease disease transmission. Such studies, however, have historically used live animals or human volunteers as a food source. What’s really needed is a high-throughput automated assay that can screen potential new mosquito repellents without the need for live volunteers. Researchers at Rice University and Tulane University have now developed a biomaterial-based platform that could be a promising step towards that goal.
“It’s a huge game changer,” says mosquito expert Dawn Wesson from Tulane’s School of Public Health and Tropical Medicine in a press statement. “If we can study how mosquitoes feed, what they do in the process of feeding, we can better understand their potential for transmitting diseases and possibly do things to stop them from feeding.”
“We hope that this platform could rapidly identify promising candidates for more effective repellents to decrease the spread of disease in the future,” says first author Kevin Janson from Rice University.
The mosquito feeding platform, described in Frontiers in Bioengineering and Biotechnology, uses biocompatible hydrogels to mimic skin. The researchers 3D printed rectangular patches of hydrogel containing a simple vascular network that can be filled with blood or other liquids. They note that the hydrogels can be printed on a large scale at a low cost and refrigerated until needed.
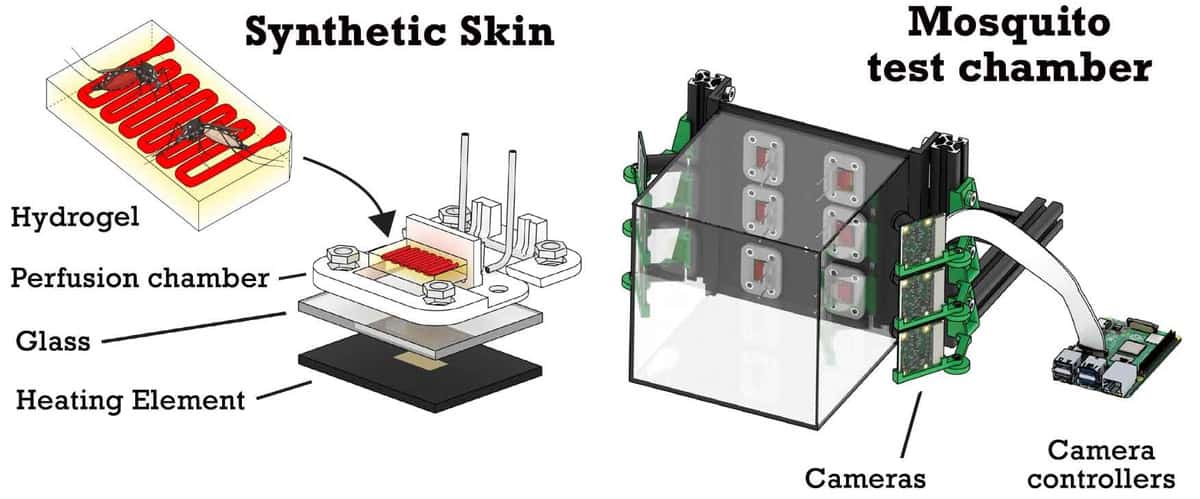
The hydrogel patches are mounted on perfusion chambers to provide a continuous supply of blood, along with a heating element for temperature control. For feeding tests, up to six of these patches are housed within a glass cage to confine the mosquitos, with a Raspberry Pi camera aimed at each patch to recordthe insects’ landing locations and feeding patterns.
Over a period of six months, Janson and colleagues collected more than 180 recordings of mosquito feeding patterns. For these experiments they filled the hydrogels with animal blood, introduced 20–30 mosquitos into the cage, and recorded 30–45 min of feeding activity.
The researchers then used these videos to train a machine-learning model to detect mosquitoes in the camera’s field-of-view. They also trained the model to identify whether a mosquito had an engorged abdomen (from feeding) or not (non-feeding).
The trained model could identify mosquitoes with 98% precision, and was able to classify abdomens as engorged or not with 96% precision. Janson notes that the machine learning model can automate experimental analysis and provide results far more quickly and consistently than a human could.
To feed or not to feed
To determine whether mosquitoes feeding on the hydrogels were attracted to the blood itself, the researchers performed experiments using three cages containing hydrogels perfused with blood, red ink or phosphate-buffered saline (PBS) as a control, all heated to 37°C. They introduced about 20–30 mosquitoes into each cage and observed their feeding behaviour.
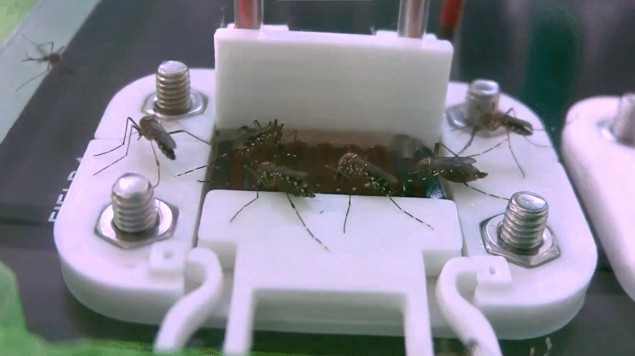
While the mosquitoes spent a substantial amount of time around all of the hydrogels, they only fed on hydrogels containing blood. This experiment confirmed that hydrogels do not inherently attract mosquitoes, and that a chemical component of blood, rather than its visual appearance, attracts the insects.
The researchers also used their mosquito feeding platform to evaluate the effectiveness of two repellents: 25% DEET and a plant-based repellent derived from the oil of lemon-eucalyptus plants (OLE). They again prepared three feeding cages, containing hydrogels coated with DEET, OLE or PBS. All hydrogels were perfused with blood heated to 37 °C.
The experiments successfully validated the repellent effects of DEET and OLE: none of the mosquitoes given repellent-coated hydrogels fed on the blood, while 13.8% of mosquitoes in the control cage did. The team notes that this relatively low feeding rate in the control is due to the small surface area of the hydrogels, which could be increased in future platforms.

AI and infrared spectroscopy identify the age and species of mosquitoes
The platform is currently optimized for laboratory mosquitoes, mostly Aedes aegypti in this study. It could, however, be adapted to analyse mosquitoes in the wild, which often exhibit different feeding tendencies. Deploying the platform in the field poses several challenges, but the researchers believe these could be addressed, for example, by using car batteries as a mobile power source and replacing blood with an artificial protein source.
Currently, the team is using the platform to investigate the viral transmission of dengue during mosquito feeding; future studies will involve malaria parasites. “Given the scale at which our hydrogels could be produced and the low cost of other components, we speculate that our platform could be adapted to support high-throughput testing in the future,” Jansen tells Physics World.