In a Best-in-Physics presentation at the AAPM Annual Meeting, E Courtney Henry introduced a CT-based technique for precision dosimetry in radioembolization
Radioembolization is a minimally invasive treatment for non-resectable liver tumours, in which yttrium-90 (90Y)-labelled microspheres are delivered into the liver’s arterial blood supply. These radioactive microspheres travel to a tumour’s distal arterial capillaries, where they deposit in the microvasculature and deliver localized radiation dose to destroy the tumour.
Dosimetry in 90Y radioembolization is currently performed after microsphere administration, using PET and SPECT to visualize the radiation emission from 90Y and determine the absorbed dose to the tumour and surrounding healthy tissue. But these imaging modalities have limited spatial resolution, which limits the dosimetry accuracy.

As an alternative, E Courtney Henry from the MD Anderson Cancer Centre and colleagues at Dalhousie University are developing a dosimetry framework based on CT imaging, which has inherently better spatial resolution than PET or SPECT.
While commercial glass- and resin-based 90Y microspheres cannot be effectively imaged using X-rays, Henry is investigating radiopaque glass microspheres, which incorporate high-Z compounds, developed by ABK Biomedical.
“Our purpose is to perform precision dosimetry in 90Y radioembolization through CT imaging of these radiopaque microspheres, and also to compare dose estimates to the liver calculated from CT to conventional PET-based dosimetry,” he explained.
The dosimetry workflow starts by converting Hounsfield units in a CT image into microsphere concentration (in mg/ml) using a calibration curve acquired from a calibration phantom with known microsphere concentrations.
Next, the microsphere distribution is scaled by the voxel volume and 90Y activity/mg to give the activity distribution (in Bq). Finally, the absorbed dose (in Gy) is calculated by multiplying the activity distribution by the mean 90Y lifetime then convolving it with a Monte Carlo-derived dose-voxel kernel.
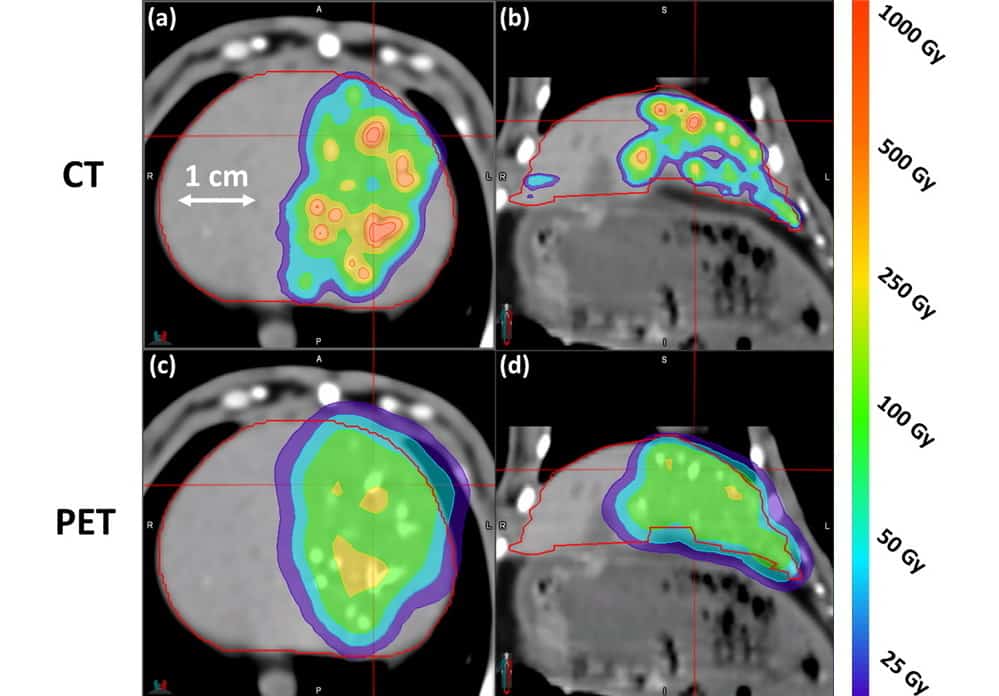
To test this approach, the researchers administered eight rabbits with a bolus of radiopaque microspheres containing 150 MBq of 90Y activity, and then performed CT and PET imaging. Henry shared images of axial and coronal slices of CT- and PET-based dose distributions in a rabbit liver.
The CT-based dose distribution appeared highly correlated with the embolized vasculature, accurately displaying the true dose heterogeneities. In addition, the dose was largely contained within the liver contour, due to the fast scanning time eliminating motion artefacts. The PET-based dose distribution, on the other hand, appeared far more homogeneous. The maximum dose to the liver calculated from PET-based dosimetry was 337 Gy, compared with 1376 Gy from CT-based dosimetry.

Targeted radiation helps treat children with inoperable liver cancer
“CT-based dosimetry in 90Y radioembolization produces a larger, more accurate estimate of mean absorbed dose relative to PET,” Henry concluded. “It reduced partial volume effects, can potentially eliminate respiratory motion effects and gave improved depiction of dose heterogeneity. This allows us to refine the understanding of the dose-response relationship and permit an individualized approach to treatment planning to improve future patient outcomes.”



