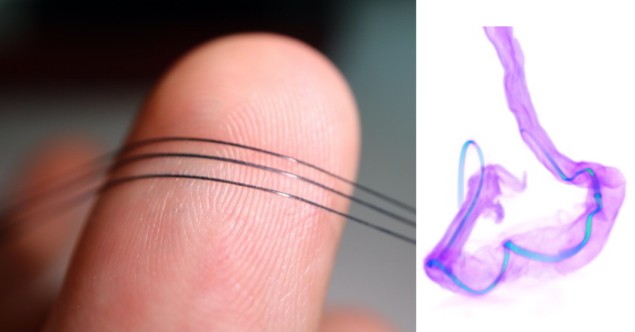
A team of Stanford University scientists has created an innovative stretchable sensor for real-time monitoring of neurotransmitter molecules in the brain and gut. The sensor, dubbed NeuroString, paves the way for a number of potential applications in the monitoring and treatment of depression, Parkinson’s disease and intestinal diseases.
The graphene-based sensor uses an electrochemical sensing technique called fast-scan cyclic voltammetry, which involves rapidly raising and lowering the voltage applied to a probe to repeatedly oxidize and reduce the target neurotransmitters, generating a neurotransmitter-specific current.
As part of their research, the researchers used the NeuroString for long-term, stable sensing of dopamine and serotonin in a mouse brain. They performed a series of experiments using optogenetic stimulation, pharmacological stimulation and behavioural assays. The results of the study, published in Nature, show that the sensor effectively detected neurotransmitter levels in the brain, while generating minimal inflammatory response.
“We then tested the sensor in the gastrointestinal tract, where its stretchability and softness conforms well to the intestinal tissue without disturbing peristaltic movement or stimulating undesired serotonin release,” says first author Jinxing Li, who carried out the work as a postdoctoral researcher in Zhenan Bao’s lab at Stanford University and is now at Michigan State University. The device provided continuous and high-fidelity monitoring of serotonin released in the gut lumen, in both a rodent model of irritable bowel syndrome and a large-animal model.
To simultaneously measure the dynamics of brain dopamine and gut serotonin, the researchers implanted some mice with NeuroStrings in both the brain and colon. When they fed the mice chocolate, the sensors detected spikes of dopamine in the brain and spikes of serotonin in the gut.
“Neurons communicate through both electricity and chemical neurotransmitters,” Li explains. “Monitoring the electrical and chemical signal communication in the nervous systems is essential for studying the brain, understanding brain diseases and developing effective therapies.”
Clinical applications
Researchers have previously made strong progress in neurotransmitter sensing through the use of genetically engineered fluorescent probes, and bioelectronic neural interfaces have been used to study wild animals and even humans. However, existing bioelectronic tools for studying neurotransmitters are limited in several ways, including the fact that they tend to be rigid and brittle, and can lead to undesired stimulation of target tissue or inflammatory responses, making them poorly suited to monitoring soft tissues.
Instead, scientists need soft bioelectronic interfaces that can monitor the natural spatiotemporal dynamics of neurotransmitters in both the central and peripheral nervous systems, without interfering with the physiology of soft and moving organs such as the brain and the gut. “These tools could ultimately enable the development of next-generation brain–machine interfaces and medical therapies that modulate neurotransmitter activity,” says Li.
The team selected graphene as the electrode material because it acts as a catalyst for the oxidation of monoamine neurotransmitters such as dopamine and serotonin. According to Li, it also has excellent electrical properties, good biocompatibility, and can withstand bending, stretching and twisting.
Using a process called laser carbonization, the team created a network of laser-induced graphene nanofibres with transition-metal nanoparticles decorated on the surface. These nanoparticles bind to the neurotransmitters and improve electron transfer, enabling the sensor to sensitively and selectively analyse neurochemistry.
The researchers then embedded the graphene nanofibre network in an elastomer matrix to make it soft and highly stretchable, while preserving the unique electrochemical properties of the nanomaterials. “The graphene nanofibres maintained an interconnected 3D conductive network even when they were deformed in the matrix,” says Li.
“NeuroString’s elastic features make it suitable for simultaneously monitoring neurotransmitter signalling in both nervous systems, and potentially addresses current technical limitations in studying the dynamics of the gut’s chemistry and interactions with microorganisms,” Li adds.
Li expects that NeuroString will be a great tool for studying gut microbe metabolites and diagnosing gut inflation, and predicts that it might also be integrated with a neurostimulator for closed-loop stimulation to treat Parkinson’s disease.
New nanosensor targets dopamine
In future work, the researchers hope to improve the sensor’s spatial resolution using micro- or nanofabrication, as well as enhance its selectivity and functionality by incorporating different probes. Eventually, they aim to integrate the sensor with wireless hardware, which should enable validation of its long-term performance in the brain and gut of larger animals.
“It might even be possible to link the sensor to a system for modulating the concentration of targeted neurotransmitters. This implanted, closed-loop system could be used to reprogram a person’s brain chemistry in real time,” says Li.