When the mice brain cells glowed, the researchers knew they were onto something. The study, from a team at Emory University School of Medicine, Atlanta Veterans Affairs Medical Center and Tzu Chi University, announces a novel, non-invasive, regenerative method called optochemogenetics that could improve stroke recovery (J. Neuroscience 10.1523/JNEUROSCI.2010-18.2019).
The second leading cause of death worldwide, according to the World Health Organization, stroke often causes structural damage and functional deficits. Research suggests that neuroprotective treatments for stroke are largely ineffective but that regenerative methods may offer the structural and functional improvements patients and their families are looking for.
“In stem cell transplantation therapy in basic or clinical research, transplanted cells are usually left in the implanted site(s) without continuous care and guiding signals to support these cells… Optochemogenetics can take the full advantage of optogenetics as well as a drug treatment,” lead author Shan Ping Yu explains.
Combining the best of both worlds
So, what is optochemogenetics? It’s a combination of two techniques – optogenetics and chemogenetics – that activate transplanted stem cells in the brain, promoting cell differentiation, growth and recovery. Optogenetics uses light to selectively activate transplanted stem cells. Because of this, it is invasive and limited by light scattering, meaning that light introduced by an optical fibre can only travel about 200 µm from the fibre tip. Chemogenetics uses chemically engineered molecules instead of light to activate cells, so that cells can be stimulated over larger brain regions. This feature is especially important because transplanted cells are mobile and capable of travelling long distances.
The researchers developed an optochemogenetics fusion protein, luminopsin 3 (LMO3), and introduced LMO3 to induced pluripotent stem-cell derived neural progenitor cells (iPS-NPCs). They then studied the behaviour of the LMO3–iPS-NPCs before injecting them into the brains of stroke-affected mice.
iPS-NPCs have three desirable properties for research: they can be directly generated from adult cells; they propagate indefinitely; and they differentiate into almost every other body cell type, including neural cells. LMO3 is special because it allows LMO3–iPS-NPCs to glow and be activated by either an external light source or a substrate called CTZ.
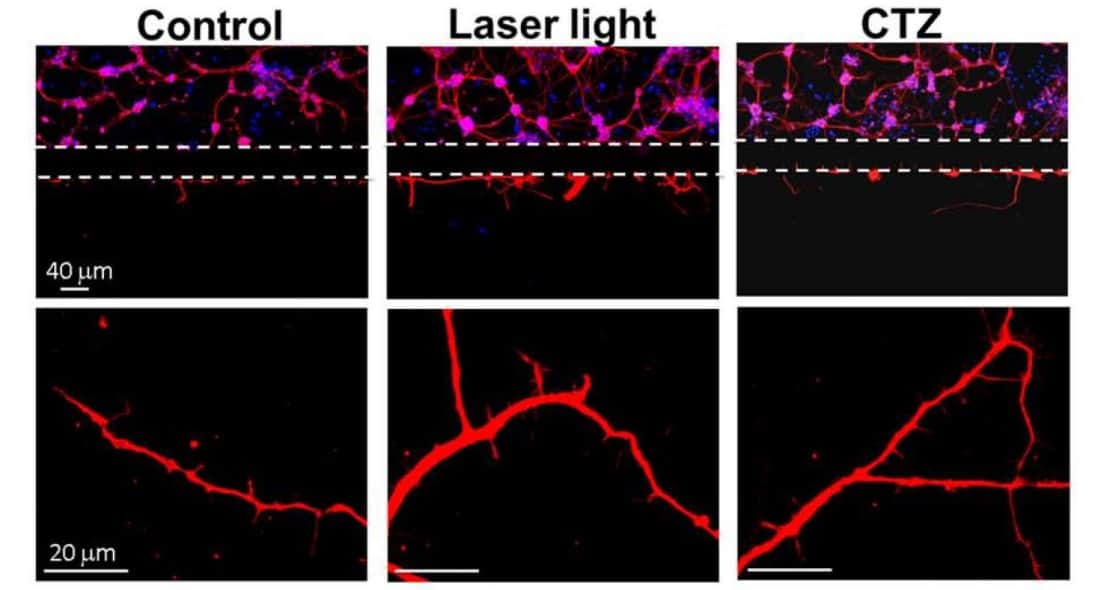
Increasing neuronal structure and connectivity
After introducing LMO3 to the iPS-NPCs, the researchers performed several experiments. They first studied the behaviour of LMO3–iPS-NPCs in vitro in three groups: LMO3–iPS-NPCs exposed to blue laser light (473 nm); to CTZ; and to no external stimulation. The researchers then used confocal microscopy to image neurites and neurite outgrowth. Western blot analysis showed that LMO3–iPS-NPCs activated by the blue light or CTZ glowed, promoted growth, and increased expressions of proteins and growth factors key to neural structure and function compared with the control group.

In the second experiment, the researchers triggered ischemic stroke in mice and injected LMO3–iPS-NPCs into the ischemic region. They monitored the status of the LMO3–iPS-NPCs using in vivo bioluminescence imaging and techniques including immunogold electron microscopy, hoping to observe LMO3 expression and the formation of synapses, a critical step for signal transmission between neural cells.
They saw that the LMO3–iPS-NPCs differentiated into mature neurons, and after digging deeper, found that the LMO3–iPS-NPCs glowed for about an hour after stimulation by intranasal injections of CTZ. Daily CTZ injections resulted in improved structural and functional recovery in stroke-affected mice. By avoiding having to cross the blood–brain barrier, CTZ appeared to have a synergistic effect on stroke recovery.
The team performed the third experiment using ex vivo brain slices and found that both CTZ and light stimulation facilitated synaptic transmission and induced neuroplasticity in forebrain slices. Further experiments are required to explain gender and age differences in recovery.
“All levels of studies – cellular, molecular, tissue, networks and behavioural – should be performed” to better understand the effects of the combined LMO3–iPS-NPC/CTZ treatment in the mouse model, and, ultimately, translate this into clinical human therapies, Shan Ping Yu says. The current research was conducted 2–3 days after ischemia was induced until 1–2 months after stroke. The fact that this is equivalent to a few human years raises hopes of a future treatment for structural and functional recovery after stroke.



