FLASH radiotherapy – an emerging treatment technique in which radiation is delivered extremely rapidly at ultrahigh dose rates – offers the potential to spare healthy tissues while effectively killing cancer cells. For proton therapy, FLASH treatments could also prove economically favourable by substantially increasing patient throughput, alongside improving treatment outcome and significantly decreasing radiation-related side effects.
With its prospects of one day revolutionizing radiation oncology, FLASH has received a tremendous amount of research attention. But before it can be routinely employed in the clinic, researchers need to understand the biomedical mechanisms underlying the FLASH effect, demonstrate that it does indeed reduce normal tissue toxicity, and characterize its impact on medical personnel and instrumentation.
Towards this goal, a team headed up by Karol Lang at the University of Texas at Austin is investigating the use of positron emission tomography (PET) to help in the transition to clinical FLASH therapy. The researchers have now demonstrated the first ever recorded PET imaging and dosimetry of a proton FLASH beam.
“We have successfully realized tests which open a new PET modality with proton FLASH beams leading to improved monitoring of irradiations and image-guided FLASH proton therapy,” the researchers write in Physics in Medicine & Biology.
PET verification
In-beam PET scanning can verify proton range by mapping positron emitters (such as 15O, 13N or 11C) generated along the beam path as the protons interact with tissues in the patient. During FLASH delivery, the instantaneous positron intensity could be up to 1000 times higher than in conventional proton therapy. In addition, the sub-second FLASH spill means that the signal does not experience the washout seen during longer beam deliveries. This strong and fast signal could enable unique monitoring of proton therapy, but also poses challenges for the PET instrumentation, which must function within an intense radiation zone.
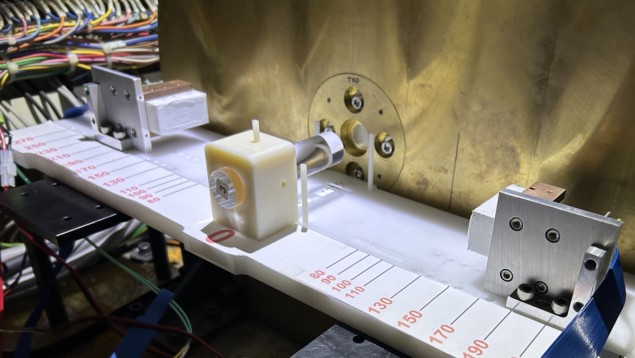
For their experiments, Lang and collaborators used the 75.8 MeV proton FLASH beam at the MD Anderson Proton Therapy Center. This beam delivers a dose rate of about 163.7 Gy/s and has an intensity of about 3.5 x 1010 protons during each 101.5 ms FLASH spill. They developed a PET “mini-scanner” using two PET modules assembled from LYSO:Ce scintillation crystals. Each module incorporates two 8 × 8 arrays of crystals, with each crystal coupled to a silicon photomultiplier.
The researchers initially characterized the proton FLASH beam using detectors known to operate well in high radiation environments. They first irradiated a PMMA block with the FLASH beam and measured the emitted radiation using a cadmium–zinc–telluride-based M400 gamma imager, which can measure annihilation gamma rays and prompt gammas at up to 140,000 counts/s.
The resulting spectra showed peaks due to positron annihilation gammas (511 keV) from induced isotopes and pair production interactions of prompt gammas, as well as prompt gammas from 12C emitted from the target. Overlaying a 2D image reconstructed from the annihilation gammas onto an optical image of the experiment provided visualization of the 2D size and range of the FLASH pencil beam in the phantom, during and immediately after irradiation.
Proof-of-principle
Following this initial assessment, the researchers assessed the PET mini-scanner’s potential for imaging and dosimetry of a FLASH beam irradiating a cylindrical PMMA phantom. They used the PET modules to measure the yield and time evolution of activated positron emitters in the phantom, which was centred on the beam axis. The system recorded results over 200 s, which included three 101.5 ms FLASH spills.
During this 200 s acquisition, the system detected 163,394 coincidences (events detected in two opposing crystal arrays within a 10 ns timeframe) in 3815 distinct pairs of readout channels. Plotting the number of observed coincidences over time revealed clear jumps in phantom activation by the three FLASH spills. However, due to the high rate of hits in the PET crystals, which the readout and data acquisition system were unable to process, the PET system readout dead-timed during each spill.
“The bottom line is that our system works well with after-spill events (due to activated positron-emitters) but requires a new approach to image in-spill prompt gammas due to excitations of a multitude of isotopes,” says Lang. “We have ideas how to do it and we are proposing it to the NIH [National Institutes of Health].”

The researchers determined the line-of-response (LOR) for each coincidence crystal pair and used these LORs to reconstruct images with a voxel size of 1.5 mm3. Despite a limited field-of-view (the setup geometry meant that only the lower crystal arrays were sensitive to back-to-back gammas emitted from the activated phantom), the images clearly showed the path of production of positron-emitting isotopes.
This ability to count events and LORs demonstrates the dosimetric capability of the in-beam PET mini-scanner. The researchers point out that that full analysis of the PET images and dosimetry is underway, but that this work offers a first glimpse of “such unprecedented possibility for a FLASH beam”.

NPL introduces absolute dosimetry for FLASH proton beams
“The main motivation for this report is to share with the public our recent results of studying FLASH beam data before more elaborate measurements can be conducted. Our results deliver encouraging news about PET imaging of FLASH beams,” they conclude.
Lang tells Physics World that the team recently submitted another publication reporting on beam exposures of six phantoms and analysis of data recorded both during the 101.5 ms spill and up to 20 min afterwards. “These unprecedented studies pave a way to a new important PET modality that will improve the overall outcome of proton therapy,” he says.