Independent researchers have found no evidence of room-temperature superconductivity in a modified form of lead apatite, dashing hopes of a technological breakthrough. The material came to public attention in July after two Korean scientists, Sukbae Lee and Ji-Hoon Kim, together with colleagues in Korea and the US, claimed it could conduct electricity without resistance at ambient pressure and temperatures. Subsequent attempts at replicating their results have come up short, however, and some experts believe the tantalizing finding may have been due to impurities in the supposedly superconducting sample.
Materials that carry current without resistance at high temperatures have long been sought after. A true room-temperature superconductor would bring major benefits, including efficient electrical grids and more powerful computers. It would also make it possible to reduce the size and cost of particle accelerators, MRI machines and other devices that rely on bulky cooling systems to keep their magnetic coils in a (low-temperature) superconducting state.
In a pair of un-peer-reviewed papers posted on the arXiv preprint server on 22 July, Lee, Kim and colleagues claimed to have produced such a material. Dubbed LK-99 after its discoverers’ initials, the material was first described as a room-temperature superconductor in April in a little-noticed paper published in the Journal of the Korean Crystal Growth and Crystal Technology. More recently, in the two arXiv papers, the team specified that the material displays four signs of superconductivity: resistance-free current flow; magnetic field expulsion and levitation (the Meissner effect); and a critical temperature and critical magnetic field below which the superconducting transition occurred.
The team also proposed an explanation, suggesting that superconductivity could arise from chemical pressure or “stress” caused by introducing copper atoms into lead apatite. Since the arrangement of these introduced impurities is crucial, the team provided a “recipe” for making the material, X-ray diffraction data on its structure and a chemical formula for the final product: Pb10−xCux(PO4)6O, where x is the concentration of copper atoms and is between 0.9 and 1.1.
First replication attempts come up short
Armed with this information, many research groups (and at least one technically-knowledgeable amateur with access to good equipment) began synthesizing their own samples. “The possibilities within the claims of Lee, Kim et al. would be a game-changer for society if they would turn out to be true, so we of course wanted to be part of the history in case of a real breakthrough,” says Ross Colman, who participated in a live online replication attempt with colleagues at Charles University in Prague, Czechia.

Initial experiments did not produce any breakthroughs. In one of the first reports, V P S Awana and colleagues at India’s CSIR-National Physical Laboratory (NPL) synthesized a sample that became weakly magnetic when placed on a strong magnet, rather than expelling magnetic field like a superconductor would. Another early replication attempt, this one by Zhiqi Liu and colleagues at Beihang University in Beijing, China, yielded a sample that behaved like a semiconductor, with a large room-temperature resistance.
Colman says these inconsistencies are partly due to the “messy” nature of the instructions for synthesizing LK-99. “Whilst the synthesis recipe is presented very simply, there are a number of inaccuracies or missing information,” he says. Examples include the dimensions of the equipment used, how the temperature should change during different synthesis stages, and even the fact that the material becomes molten at 925 °C. “A more detailed description would have prevented a lot of guesswork,” he says.
A door opens a crack…
On the theoretical side, things were slightly more encouraging. A team led by Xing-Qiu Chen of China’s Shenyang National Laboratory for Materials Science calculated that a material with the formula Pb10−xCux(PO4)6O would contain electronic structures known as flat bands at the Fermi level, which is the highest energy level that an electron can occupy at 0 K. These flat bands can be a hallmark of superconductivity.
Independently, Sinéad Griffin, a staff scientist at the Lawrence Berkeley National Laboratory in the US, came to a similar conclusion: swapping copper for lead in the appropriate place within Pb10−xCux(PO4)6O produces flat bands. Less promisingly, Griffin calculated that an experimentally easier substitution has no such effect. “This result hints to the synthesis challenge in obtaining Cu substituted on the appropriate site,” she wrote.
…and then slams shut
Despite this caveat, the flat-band results were greeted with elation among LK-99’s growing army of fans on social media. When Griffin posted her paper on Twitter/X, accompanied by a “mic drop” GIF featuring former US president Barack Obama, the responses included “This is so badass” and “Incredibly based tweet”.
Over the next fortnight, though, the replication failures continued, and the air began to leak out of the hype bubble. A second effort by the Indian NPL team used purer precursor materials and produced a sample with X-ray diffraction peaks that more closely matched those in the original arXiv papers. Alas, this new sample was not a superconductor either. It was diamagnetic, becoming magnetized in the opposite direction to the applied field.

This is important, because one of the strongest pieces of evidence in favour of room-temperature superconductivity in LK-99 was the material’s ability to levitate when placed atop a strong magnet under ambient conditions. The Korean team interpreted this as being due to the Meissner effect, but diamagnetic objects (including frogs and strawberries) will also levitate if the magnetic field is strong enough.
Another alternative explanation for LK-99’s levitation came from Shuang Jia and colleagues at Peking University in Beijing. Although they persuaded “some small flaky fragments” of their synthesized sample to levitate, these levitating fragments “ubiquitously contain weak yet definitive soft ferromagnetic components”. Ferromagnetism, they wrote, can explain levitation in LK-99 without recourse to superconductivity.
A fuller picture emerges
For Andrei Bernevig, a condensed-matter theorist at Princeton University, US, the varying results and associated hype are a source of frustration. “A lot of the stuff early on was rushed and statements from all sides were unchecked,” he tells Physics World. “The social media, memes, etc., have been completely detrimental to progress in this field in my view…I hope we never do science like this again.”
To provide concrete answers, Bernevig and his Princeton colleague Leslie Schoop, together with collaborators in Spain, Germany and the University of Oregon, US, focused on a different question. Rather than investigating whether LK-99 exhibited signs of superconductivity, they began by asking: just what is LK-99, anyway?
After synthesizing their own sample, the team performed X-ray diffraction measurements on the best crystal in the batch. This crystal turned out to contain at least three different components. “The recipe is simple, but it does not result in a single-phase material,” explains Schoop, a materials chemist. “When a sample consists of multiple materials, as LK-99 seems to, it is difficult to get the exact same results in different labs.”
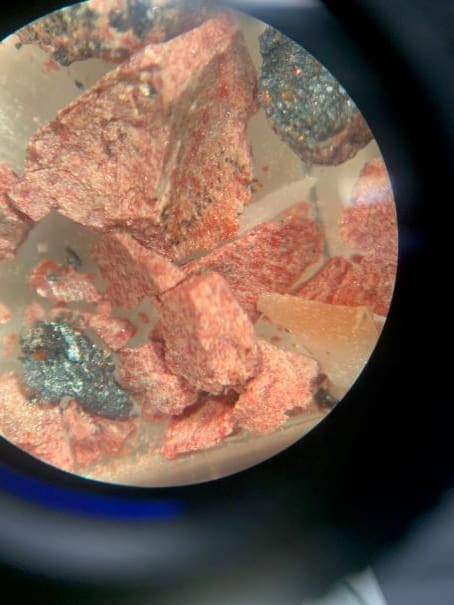
At first glance, this conclusion might seem to support the hypothesis put forward by online LK-99 fans who suggested the replication failures were due to incorrectly-synthesized samples. Alas, theorists in the Princeton team calculated that in a material with the “correct” structure, the flat bands that inspired so much excitement are localized and thus, in effect, the wrong type. “These localized flat bands tend to give rise to magnetism in LK-99 (for the assumed structures) instead of superconductivity,” explains team member Jiabin Yu, a postdoctoral researcher in condensed-matter theory at Princeton.
Other calculations by the same team showed that copper atoms are unlikely to enter the structure of LK-99’s precursors in concentrations high enough to affect its properties. This suggests that the Korean team’s explanation for superconductivity is incorrect. It also casts doubt on the material’s proposed structure, with consequences for theorists as well as experimentalists. “If the structure of ‘LK-99’ is different from the assumed ones, then we currently cannot make any reliable claims about the superconductivity,” Yu says.
The role of impurities
A further insight came from Wei Wu, Jianlin Luo and colleagues at the Beijing National Laboratory for Condensed Matter Physics, China. Like the Korean researchers, they observed a sharp “superconducting-like” transition in the resistivity and magnetic susceptibility of LK-99 at temperatures a little below 400 K.
However, they suggest that this could have arisen from Cu2S impurities in the original sample, which the Korean researchers acknowledge were present. Cu2S undergoes a structural phase transition at around 385 K, and the Beijing researchers found that this phase transition produces a sharp drop in the resistivity of an LK-99/Cu2S mixture. This, they say, may be what the Korean team saw.
“If there’s a simple alternative explanation for the results, there’s no reason to consider the extraordinary claim of room-temperature superconductivity anymore.”
Michael Fuhrer
When Physics World put these questions to members of the team, Lee, the corresponding author for the first arXiv paper, did not respond. Hyun-Tak Kim, a physicist at the College of William and Mary in the US and the corresponding author for the second arXiv paper, declined to comment because he has submitted the paper to a journal, and will address criticisms in his response to a reviewer’s report.
“The final nail in the coffin”
Absent further developments, Michael Fuhrer, a condensed-matter physicist at Monash University, Australia, who has been following the replication attempts, calls Wu and Luo’s result “the final nail in the coffin” for LK-99 as a room-temperature superconductor. Together with the ferromagnetism and diamagnetism reported elsewhere, Fuhrer says it shows that the Korean team’s findings can “very likely be explained” by the presence of impurities in their sample. “If there’s a simple alternative explanation for the results, there’s no reason to consider the extraordinary claim of room-temperature superconductivity anymore,” he tells Physics World.

Have scientists in Korea discovered the first room-temperature, ambient-pressure superconductor?
Colman is slightly more optimistic. “There remains a spark of hope that the observations of superconductivity are still real, if related to a very specific impurity in the Korean samples,” he says, “but tracking down the truth of the observations may be a very difficult process. Experimental reproduction of the properties is impossible if only a single grain in a multigram batch shows the properties that you are interested in.”
Still, Fuhrer doesn’t think the scientific community should judge the team harshly. “Science isn’t a court of law, and we’re unlikely to get ‘proof’ that the original LK-99 samples don’t contain any superconductor,” he says. Instead, the failed replication attempts “simply show that the results are more likely to be explained another way…I think this was a genuine example of competent scientists who believed they were right but got fooled in a rather subtle and surprising way.”